Opportunity
Changing the Paradigm for People Living with COPD
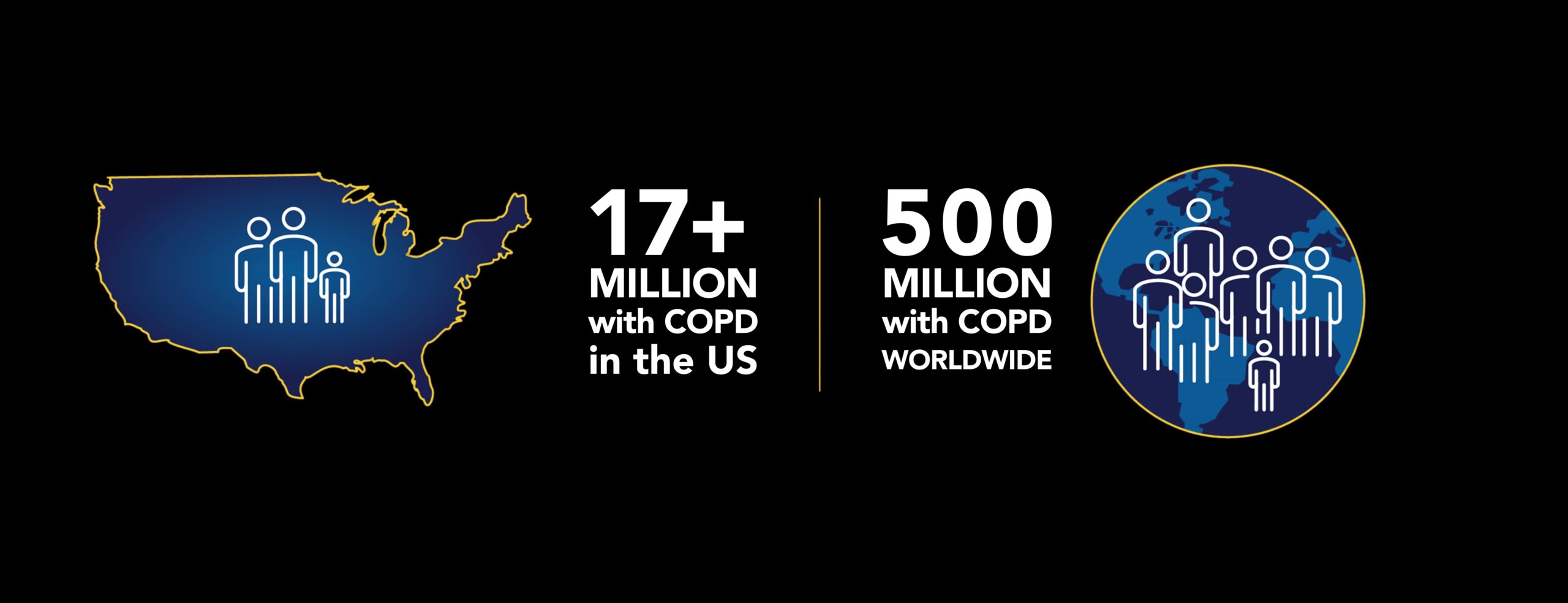
COPD is among the top 5 causes of death in the United States and is projected to be the world’s #1 cause of death within 15 years. More than 17 million Americans are living with COPD, and they experience hundreds of thousands of hospitalizations each year. Globally, there are an estimated 500 million people living with COPD, including 44 million in Europe and over 100 million in China.
The health and economic impacts of COPD are staggering. It is clearly time for innovation.
Although there is currently no way to reverse the chronic lung changes of COPD, patients can live productively and happily if they avoid acute deteriorations that rob them of breath and put them on a trajectory for ever-worsening disease.
The main cause of these deteriorations are viral respiratory infections, most commonly rhinovirus. Unfortunately, until now (unlike for COVID and influenza), there have been no anti-viral treatments (like Tamiflu or Paxlovid) for rhinovirus. Altesa’s mission is to change this unfortunate situation.
Without an antiviral medicine, patients are forced to manage the consequences of rhinovirus infections by using more inhalers, taking steroids, or starting oxygen therapy. Viral infections also can lead to bacterial infections, so patients often receive antibiotics whether there is a proven bacterial infection or not. And most recently, about one third of COPD patients qualify to receive monthly immune modifying injections, which have been shown to reduce the consequences of viral infections in some patients.
Altesa aims to treat the main cause of exacerbations, not the downstream consequences. By providing a safe and effective medicine that stops rhinovirus in its tracks, we aim to empower COPD patients by enabling them to treat the infection before it can lead to potentially devastating life-long downstream consequences.
Why Altesa?
Altesa’s lead medicine, Vapendavir, is the only drug in human clinical trials that directly targets rhinovirus. Vapendavir has been administered safely to over 700 normal health volunteers and participants with COPD or asthma. These clinical trials have demonstrated that vapendavir is well tolerated, and has potent antiviral activity against all rhinovirus families.
Although rhinovirus is the primary cause of many illnesses, from the common cold to asthma in children to severe infections in transplant patients, our first objective is to improve the lives of people living with COPD by treating their number one cause of acute exacerbations.
Unless there is a new approach for COPD, studies indicate that, by 2038, people with COPD in the United States will suffer:
Altesa’s goal is straightforward: by identifying high-risk patients with early rhinovirus infections and providing safe and effective oral treatment, we can prevent respiratory deterioration and preserve the health status of people with COPD and in the future many other chronic lung conditions. Altesa is now conducting large multicenter clinical trials in the United States and the United Kingdom to advance vapendavir so that it may become available for general use as soon as possible.
