Opportunity
Changing the Paradigm with Chronic Obstructive Pulmonary Disease (COPD)
The world has many more viruses that cause suffering and death in vulnerable people than we have antiviral treatments to fight them. New antiviral medicines present an enormous opportunity to improve health outcomes for people disproportionately impacted by common viruses due to health conditions, age, or weakened immune systems.
The most effective treatments will quickly stop the progressions of viral infections and prevent hem from causing long-term damage, especially to the lungs.
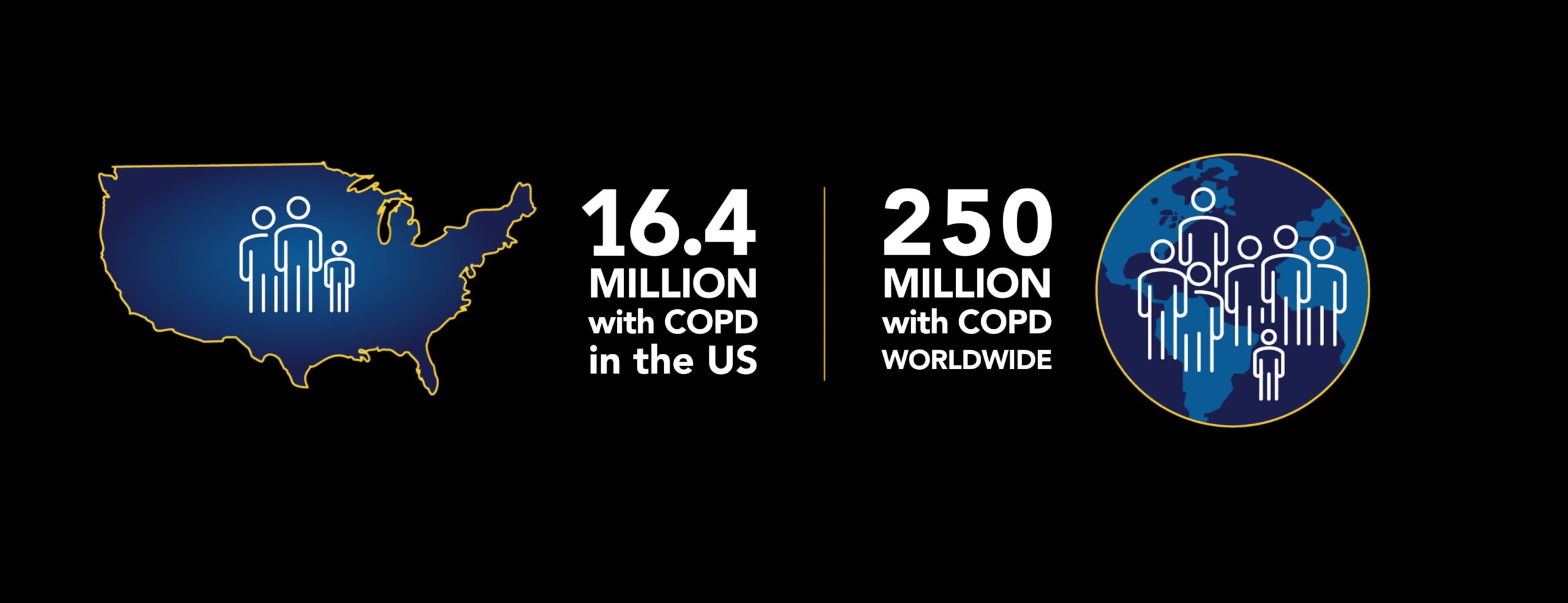
COPD is generally among the top 5 causes of death in the United States and is projected to be the world’s #1 cause of death within 15 years. More than 16.4 million Americans (6.6% of U.S. adults) and over 44 million Europeans are living with COPD. Estimates suggest over 100 million people in China suffer with COPD. Globally, there are an estimated 400 million people living with COPD.
For people living with this condition, “catching a cold” can become a serious illness resulting in diminished lung function, reduced quality of life, financial hardship, hospitalization, and even death. Significantly, the incidence of COPD in women in the United States, especially in the sunbelt, is accelerating at a rate higher than ever previously observed.
Those with fewer financial resources or other social barriers to health care are often diagnosed with COPD later in the course of the disease, when symptoms are more severe. As a result, especially for African Americans, the outcomes of COPD are generally poorer. (Mamary 2018, Eisner 2011, Woo 2021).
Altesa’s clinical program will address these disparities in vulnerable populations by ensuring broad representation in our clinical enrollment and development plans while supporting programs and national initiatives that aim to reduce disparities in diagnoses.
Altesa’s value lies in its unique strengths:
Vapendavir, an oral medication Phase 2 clinical drug, has been administered safely to over 700 normal health volunteers and participants with COPD or asthma. Clinical trials have demonstrated that it is a broad-spectrum treatment (capsid inhibitor) with potent activity across all rhinovirus families.
Common viral respiratory infections may have serious consequences, including death, especially for those who are vulnerable due to underlying health conditions, age (both young and elderly), or weakened immune systems. Medicines that specifically target these viruses could make the difference between full recovery and serious health consequences . Altesa’s objective is to develop these life changing medicines to be available to the right patients at the right time.
Our first goal: treating rhinovirus infections in time to prevent long-term consequences in people with chronic obstructive pulmonary disease (COPD).
More than 16.4 million Americans, or 6.6% of U.S. adults, have been diagnosed with COPD. COPD is the third leading cause of death worldwide, according to the World Health Organization, and it is expected to become the #1 cause of death worldwide within 15 years. Those with limited financial resources, especially those in urban areas, are often diagnosed later in the disease, which is associated with more severe outcomes.
Viral infections are the primary cause of acute deteriorations in lung function and overall health (known as “exacerbations”) for people living with COPD. Among viral causes, rhinovirus—the predominant cause of the common cold—is the most frequent culprit causing these infections.
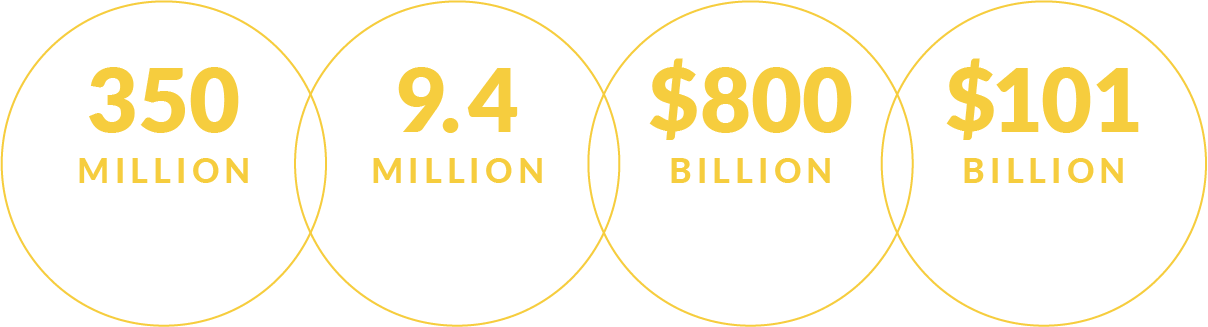
Studies indicate that, by 2038, people with COPD in the United States will suffer:
Altesa’s mission is straightforward: By identifying high-risk patients with early rhinovirus infections and providing safe and effective oral treatment, we can prevent respiratory deterioration and preserve the health status of people with COPD. Altesa is now sponsoring clinical trials in COPD patients to prove this mission.
