Pipeline
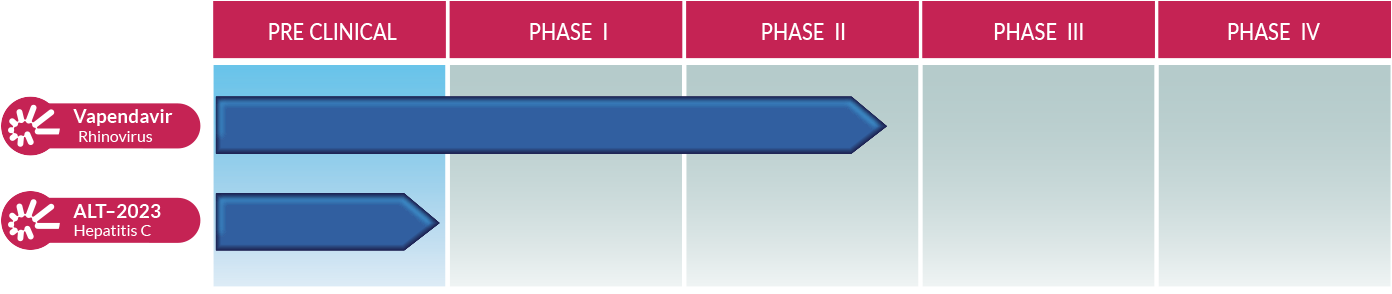
Vapendavir, a broad-spectrum capsid inhibitor (prevents the virus from entering human cells and also prevents the virus from reproducing), exhibits potent activity across 97% of rhinoviruses and other respiratory enteroviruses. It is our most advanced pipeline drug. Altesa holds exclusive global rights to develop, manufacture, and commercialize vapendavir. This novel antiviral treatment, taken in pill form, is in a Phase 2b clinical trial sponsored by Altesa.
ALT-2023 is a late preclinical stage drug licensed from Emory. This molecule is a broad-spectrum nucleoside analog (prevents effective viral replication). It shows high potency against the hepatitis C virus and has activity against most enteroviruses and flaviviruses (causing dengue, yellow fever, West Nile disease, Zika, and other serious illnesses).
